The UK’s medicines watchdog says it has found 30 cases of rare blood clots in people who have had the Oxford-AstraZeneca Covid vaccine.
The Medicines and Healthcare products Regulatory Agency (MHRA) says the risk of having this type of clot is “very small”.
The UK cases were out of more than 18 million doses given as of 24 March.
Fears over clots have led nations such as the Netherlands, Germany, France and Canada to restrict the jab’s use.
The pharmaceutical company AstraZeneca has said international regulators had found the benefits of its jab outweighed risks significantly.
The MHRA, the European Medicines Agency (EMA) and the World Health Organization (WHO) have also said the benefits of taking the vaccine outweigh any risks.
The MHRA said it had received 22 reports of cerebral venous sinus thrombosis (CVST) – where a blood clot forms in the brain – and eight reports of “other thrombosis events with low platelets [the cells involved in clotting]” following use of the Oxford-AstraZeneca jab, out of a total of 18.1 million doses given up to and including 24 March.
The watchdog said the benefits of the Oxford-AstraZeneca vaccine in preventing Covid-19 infection and its complications continued to outweigh any risks, and urged the public to accept the jab when offered it.
In response to the MHRA’s data, Prof Adam Finn, a member of the Joint Committee on Vaccination and Immunisation (JCVI), insisted taking the vaccine was “by far the safest choice” to minimise the risk of serious illness or death from Covid-19.
He said: “The extreme rarity of these events in the context of the many millions of vaccine doses that have been administered means that the risk-benefit decision facing people who are invited to receive Covid-19 vaccines is very straightforward: receiving the vaccine is by far the safest choice in terms of minimising individual risk of serious illness or death.”
Meanwhile, the MHRA said there had been no reports of blood clots following use of the Pfizer-BioNTech vaccine.
As of 21 March, an estimated 10.8 million first doses of the Pfizer-BioNTech jab had been administered in the UK, the regulator said. It added that around 2.2 million second doses, mostly the Pfizer-BioNTech vaccine, had been administered.
More than 31.3 million people in the UK have received a first dose of a coronavirus vaccine, and more than 4.9 million have had a second dose, according to Friday’s daily government figures.
The Netherlands is the latest country to restrict the rollout of the Oxford-AstraZeneca jab, after saying on Friday that it was suspending its use for people under the age of 60.
The Dutch health ministry said the precautionary measure was taken after five reports of blood clots in combination with low platelet counts in women between the ages of 25 and 65.
All appointments for those under-60s scheduled to receive the jab have been cancelled with immediate effect. The suspension will remain in place until 7 April when the EMA is expected to issue new guidance.
About 400,000 Oxford-AstraZeneca injections have been given in the Netherlands, it added.
Earlier this week, Germany said it was suspending routine use of the jab for people below the age of 60 due to fears over a link with blood clots.
It has reported 31 CVSTs and nine deaths out of 2.7 million people vaccinated. Almost all the cases are reportedly in younger and middle-aged women.
The Netherlands and Germany were among several European countries which briefly suspended use of the jab last month pending an EMA review into the possible link to blood clots.
When the EMA declared the vaccine “safe and effective”, they resumed its use while investigations continued.
France already limits use of AstraZeneca to those aged over 55 and on Monday, Canada recommended immediately suspending use of the jab in people aged below 55 following reports in Europe.
The EMA, which has assessed data from around the world, estimates there is around a one in 100,000 risk of a CVST in people under the age of 60 who have been given the Oxford-AstraZeneca vaccine.
The organisation’s head of safety monitoring, Dr Peter Arlett, said that was “more than we would expect to see”.
James Gallagher, the BBC’s Health and Science correspondent, says that it is uncertain what the background rate of these blood clots in the brain truly is.
Estimates vary from around two cases per million people every year to nearly 16 in every million in normal times and coronavirus itself may be causing them too.
CVSTs are inherently more common in younger women and taking the pill increases the risk. So the natural risk levels – whether people are vaccinated or not – could have a role too.
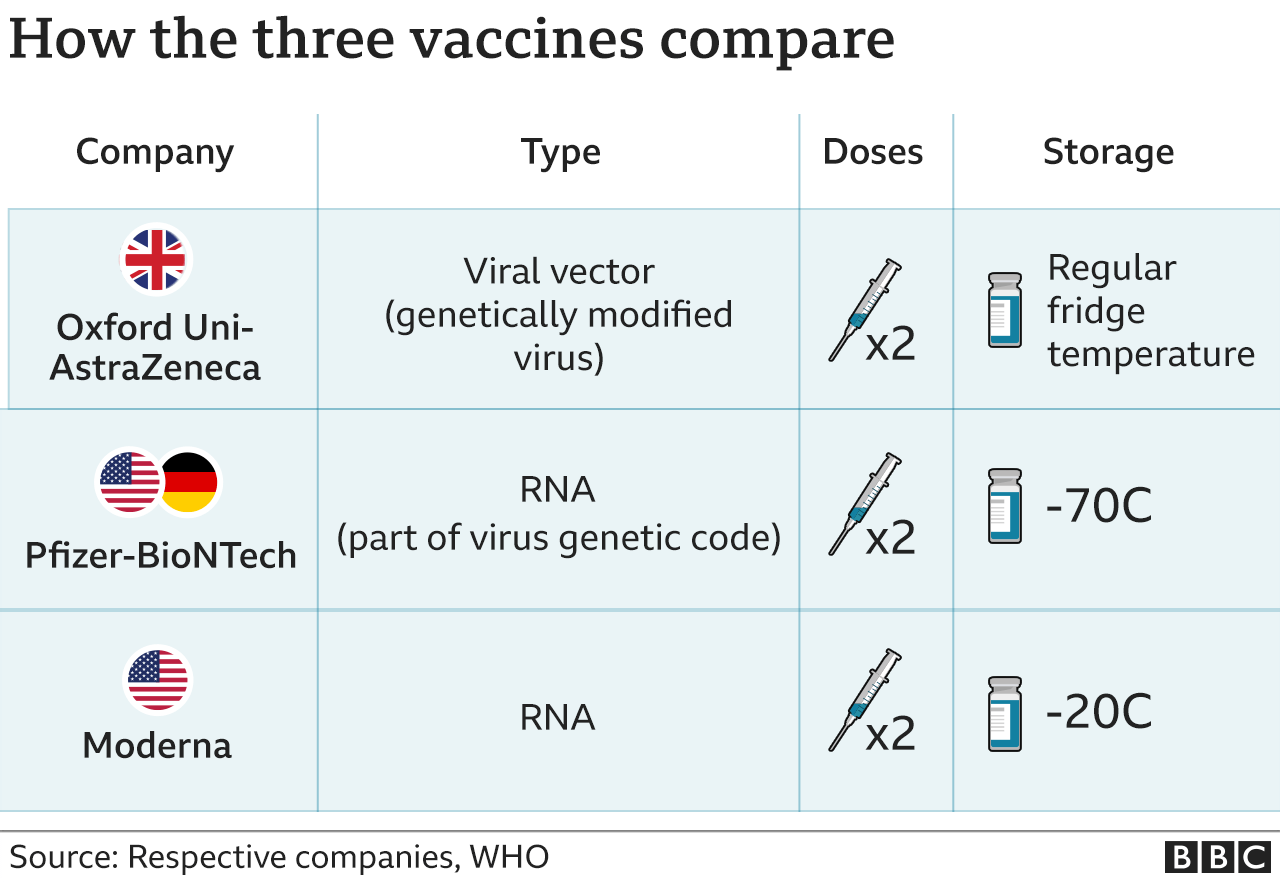
Two vaccines – developed by Pfizer-BioNTech and Oxford-AstraZeneca – are currently being used in the UK to protect people against Covid-19.
The first doses of a third vaccine – from Moderna – are due to arrive in the UK later this month. Like the Pfizer-BioNTech and Oxford-AstraZeneca jabs, Moderna’s is given in two doses several weeks apart.
The UK has ordered seven vaccines and expects to receive 407 million doses – more than enough for every adult to receive two.
The aim is to vaccinate everyone aged 18 or over in the UK with one dose by the end of July – but children could receive a vaccine in the long term if they are shown to be safe.